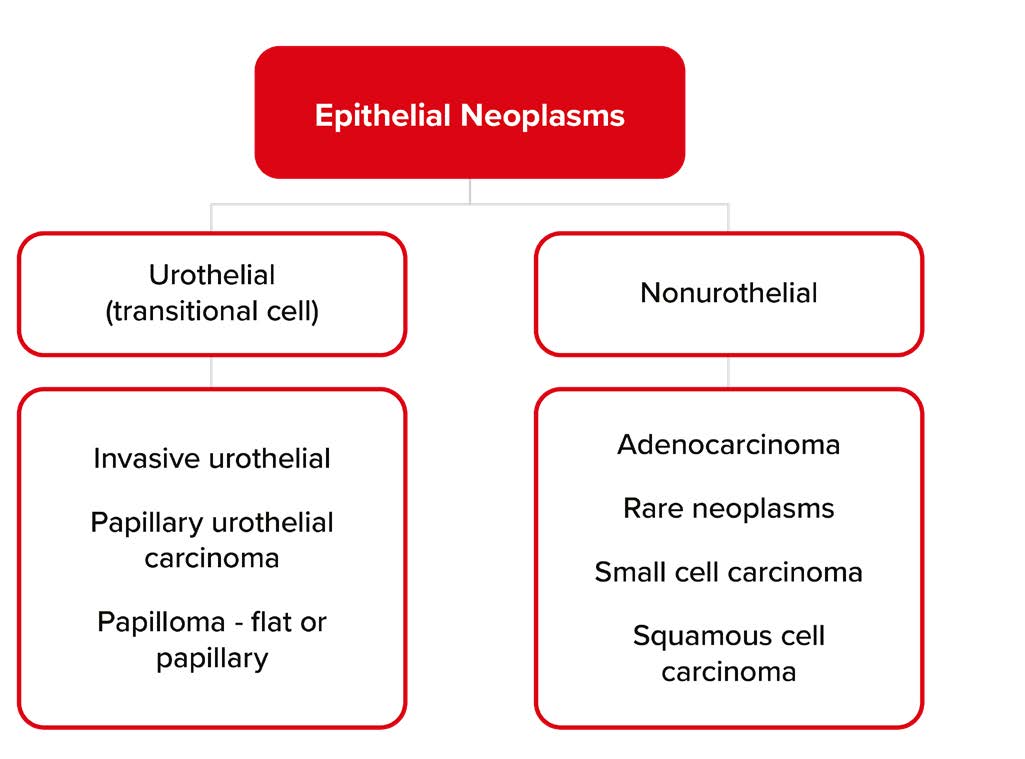
Symptoms and Diagnosis
Painless hematuria is the characteristic presenting sign or symptom associated with bladder cancer. It can be microscopic or grossly visible. Dysuria, urinary frequency, and urinary urgency are other common presentations. Diagnostic evaluation follows a risk-stratified approach that typically includes urinalysis demonstrating >3 RBC/ HPF, renal ultrasound, and cystoscopy, the last of which is considered the gold standard test.
Urine cytology can be used as an adjunct to cystoscopy, but it has low sensitivity for diagnosing low-grade tumors. This low sensitivity has resulted in the development of tests that can detect multiple urine-based tumor biomarkers, which have numerous potential applications in the detection of urothelial bladder cancer. These tests target markers such as the differential expression of DNA, RNA, cellular markers, and tumor-related proteins.
Urine-based tumor markers have been found to have greater sensitivity than urine cytology for detection of low-grade tumors, but to be inferior to urine cytology with regard to specificity. False positive tests can also occur, especially in situations such as concomitant UTI, renal calculi, or prior intravesical treatment. One study found that urinary biomarker tests miss 18% to 43% of patients with bladder cancer and yield false-positive results in 12% to 26% of individuals who do not have bladder cancer.6
Although this lack of specificity has limited the definitive clinical application of these biomarker tests, numerous potential applications still do exist.7 For example, urine biomarkers could assist in the initial diagnosis of bladder cancer by risk stratifying which individuals should undergo subsequent cystoscopy and imaging evaluation of the genitourinary tract. Similarly, biomarkers could aid in the surveillance of non-muscle-invasive bladder cancer (NMIBC) by serving as an adjunct to urine cytology to help detect the presence of low-grade tumors that might otherwise be missed, or cystoscopy to potentially serve in lieu of the procedure altogether.
Staging
Bladder cancers can be divided into non-muscle-invasive and muscle-invasive disease. Pathologic staging of bladder cancer is contingent on the magnitude of tumor invasion into deep layers of the bladder. NMIBC accounts for 80% of newly diagnosed bladder cancers, including tumors in stage 0 subgroups Ta (70%-75%) and Tis (5%-10%), and the stage I subgroup T1 (20%-25%).8
NMIBC can be further stratified into low- and high-risk categories, with varying degrees of risk for progression and recurrence. According to the 2021 European Association of Urologists (EAU) guidelines, low-risk tumors are solitary, low-grade (Ta), less than three centimeters in diameter, and not carcinoma in situ. High-risk tumors include carcinoma in situ (Tis), high- grade disease, stage T1 lesions, and low-grade (Ta) tumors that are either multiple, recurrent, and/or large (more than 3 cm in diameter). Low-risk tumors have a 0%-4% risk of progression that increases to 30%-40% for high-risk lesions. It is estimated that 31%-78% of NMIBC cases recur within five years of diagnosis, a rate far surpassing that of all other cancers.9
Muscle-invasive disease, characterized by malignant extension into the detrusor muscle, includes tumors determined to be stages II through IV.
Treatment
Management of each type of bladder cancer differs significantly and poses unique challenges. Radical cystectomy, with or without platinum-based neoadjuvant chemotherapy, is the treatment of choice for surgically amenable muscle-invasive disease but carries a high morbidity risk (60%) and a near 3% risk of mortality.10 Conversely, transurethral resection of a bladder tumor (TURBT), with or without intravesical chemotherapy or immunotherapy, is preferred for non-muscle-invasive disease.
Tumors with low risk for recurrence benefit from a single immediate instillation of one of the two intravesical chemotherapeutics, gemcitabine or mitomycin C, following TURBT to help eradicate residual disease. High-risk NMIBC requires a restaging TURBT six weeks following the initial procedure (given the up to 50% risk of understaging), followed by adjuvant immunotherapy with intravesical bacille-Calmette Guerin (BCG). BCG, the live attenuated form of mycobacterium bovis, is the most widely used intravesical therapy for bladder cancer. Treatment courses often span one to three years for intermediate to high-risk tumors and involve a robust induction and maintenance schedule.11
For more than two decades, BCG immunotherapy has been the treatment of choice for individuals with high-risk NMIBC, especially carcinoma in situ, because it delays tumor progression, reduces the need for cystectomy, and improves survival. BCG does have limitations, however, as recurrence rates for cases where it is used approach 40% at two years, treatment failure occurs in 30% to 50% of cases, it can be poorly tolerated due to adverse side effects, and there may be diminished immunologic response over time.12
Challenges and Advances
Since 2019, BCG has been in short supply worldwide due to an unforeseen reduction in the number of pharmaceutical manufacturers in the market, leaving affected individuals with suboptimal treatment options depending on the severity of disease.13 Since then, the investigative focus has shifted to biomarkers to target responsiveness to therapy. In the past few years, such alternative regimens have been developed and successfully implemented, including the use of intravesical chemotherapies mitomycin C and gemcitabine, sequential gemcitabine/docetaxel, and even initial radical cystectomy in certain high-risk situations.
Therapeutic options for individuals with NMIBC are evolving to include gene therapy, immune checkpoint inhibitors, targeted therapy, and antibody-drug conjugates (ADCs), which are a monoclonal antibody chemically linked to a drug.
Adstiladrin (nadofaragene firadenovec), one such ADC, is a recombinant nonreplicating adenovirus vector with complete response rates of up to 53%, and was approved by the U.S. Food and Drug Administration in early 2023 for BCG-unresponsive NMIBC.14, 15
Three immune checkpoint inhibitors have also been approved for individuals with locally or advanced metastatic bladder cancer. One of these treatments, Keytruda (pembrolizumab), approved in 2020, is a programmed cell-death protein 1 (PD-1) inhibitor that demonstrated a 41% response rate for BCG-unresponsive carcinoma in situ in the KEYNOTE-057 trial.16, 17 There is a pending phase III evaluation of pembrolizumab plus CG0070, an oncolytic virus therapy, following phase II trial results showing complete response in 85% of individuals with BCG-unresponsive NMIBC.18
Molecular profiling is being performed to predict which subtypes of NMIBC respond to BCG, and multiple trials are also underway to investigate alternative therapies for BCG-unresponsive bladder cancer. A recent study uncovered three distinct BCG response subtypes, one of which had a lower recurrence-free and progression-free survival, thus allowing for more targeted treatment for people unlikely to respond to BCG.19
Conclusion
Bladder cancer is a disease recognized for its high propensity for recurrence. NMIBCs, the majority of cases, often require prolonged and costly treatment. Cystoscopy remains the gold standard for diagnosis, but the expanding availability of urine tumor biomarkers offers a potential adjunct in both the identification of bladder cancers as well as ongoing surveillance. The evolution of novel therapeutics, such as gene therapy or immune checkpoint inhibitors to address BCG-unresponsive disease and the ongoing BCG shortage, is also providing greater opportunities for individuals to achieve lasting treatment response while postponing or even avoiding radical cystectomy. Such responses could pose a more favorable underwriting outlook.