As we enter year three of the COVID-19 pandemic, mortality and morbidity has changed substantially and for the better since the virus’s early days.
The vaccines introduced just over a year ago are proving a highly effective defense against severe disease. Successful options for treatment have also been increasing, but at this point they still number just a few, and not every option works for every variant. In this brief, the latest thinking around risk stratification as well as treatment will be reviewed in the context of health insurance claims, and what these may mean for health claims practices.
Testing
The two main types of tests used today to determine the presence of SARS-CoV-2 are reverse transcription polymerase chain reaction (RT-PCR) tests and antigen tests. RT-PCR tests replicate DNA samples obtained from nasal/throat swabs or saliva to determine if the virus is present in an individual’s body, and antigen tests use swab or saliva samples to determine if specific proteins originating from the virus are present.
The current gold standard for COVID-19 testing is RT-PCR, which was the first COVID-19 detection test used as a preferred test due to its high sensitivity and specificity. Results can be obtained within a few hours to a few days depending on the capacity and capability of the laboratories processing the tests. Antigen tests are also highly useful, as they can be administered at home, produce rapid results (sometimes in as little as 15 minutes), and are less expensive than RT-PCR. Antigen tests are best used in outpatient settings with patients who present with COVID-19 symptoms, or who have had contact with positive individuals and need to determine their own infectious state.
Testing for COVID-19 with RT-PCR is increasingly being incorporated into routine hospital clinical practices, especially prior to surgical procedures and when other patients in a ward test positive for the virus. It is also becoming standard practice across populations in communities experiencing outbreaks.
On the basis that these two tests now represent appropriate clinical practice, we recommend insurers cover these tests when reimbursing the cost of otherwise covered treatment.
There is a third type of test – the antibody or serology test – which is not used for diagnostic purposes. Rather, its purpose is to reveal the presence or levels of antibodies within the body so as to determine if the individual had had COVID-19 in the past. As the test is not considered medically necessary for a diagnosis, we do not recommend covering it.
Imaging
For a symptomatic patient, once they have tested positive for COVID-19, the initial step would be to conduct a general history and physical examination, which would include taking measurements of heart rate, blood pressure, and oxygen saturation. The next step is generally obtaining an image of the lungs to determine the infection’s current radiologic severity. Chest x-rays can show whether lung changes characteristic of COVID-19, such as the typical ground glass appearance, interstitial thickening, and parenchymal consolidation, have occurred. However, in cases diagnosed early in the virus’s trajectory, a chest x-ray may not be able to detect these changes. In such cases, a CT scan of the thorax may be indicated as it can detect early changes in the lungs, which would strengthen a case for hospital admission and close monitoring.
If chest x-rays or CT scans are unavailable or under-resourced due to geographic location, a lung ultrasound, which is more generally available, can also identify certain characteristic pulmonary changes of COVID-19, such as a thickened, irregular, or discontinued pleural line and B-lines.
These imaging studies, when used as indicated, should be covered.
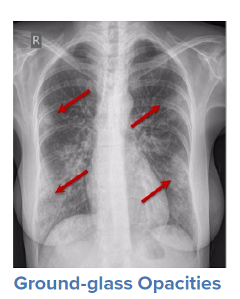
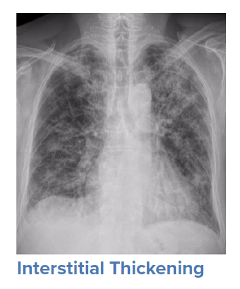
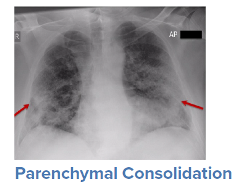
Source: WHO
Hospital Admissions
Early in the pandemic, hospital admission criteria were dictated by governmental health authorities, and sometimes required even asymptomatic individuals testing positive for COVID-19 to be admitted or at least quarantined in designated health centers. Health officials in most countries now recognize that mild to moderate COVID-19 can be managed on an outpatient basis, with exceptions depending on the patient’s potential for greater severity.
The most recently updated guidelines from the World Health Organization’s (WHO) Guidelines Development Group (GDG), published January 2022, provides criteria for three levels of severity for patients with confirmed COVID-19:
Figure 1
Patients with confirmed COVID-19
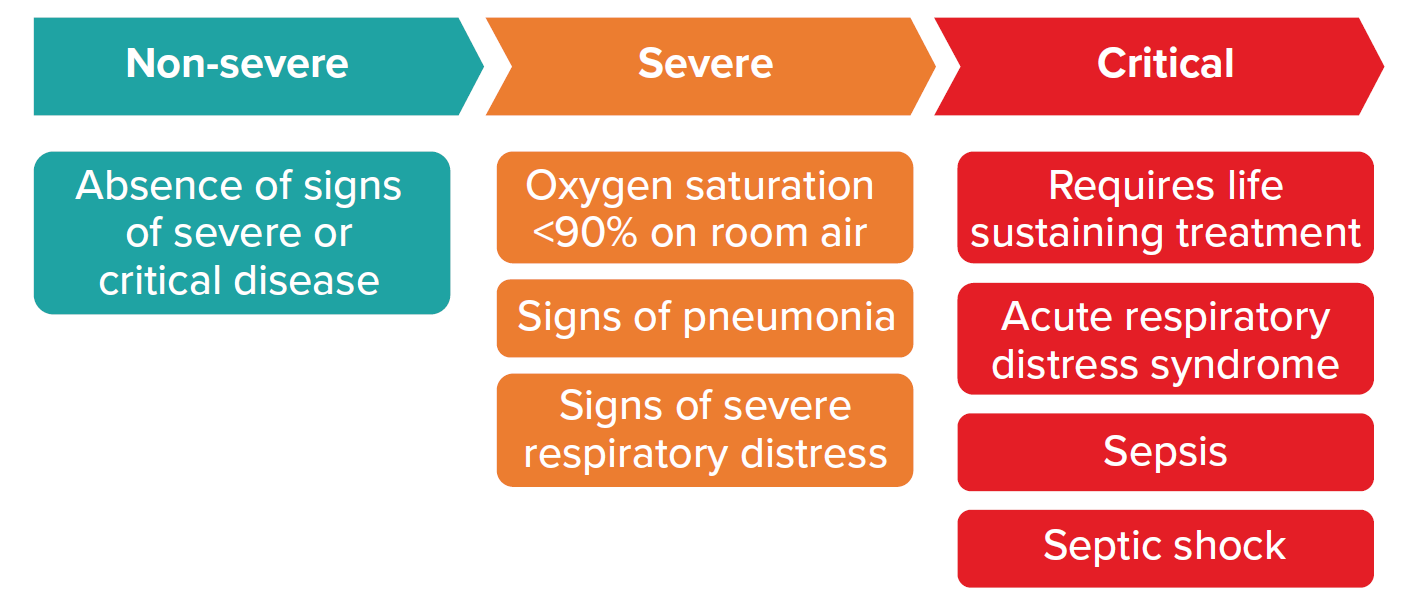
Source: World Health Organization. Therapeutics and COVID-19. 2022 Jan 14. p. 9.
As a general rule, non-severe COVID-19 cases are able to be managed in an outpatient setting. However, patients at risk of progressing to severe disease should be considered for hospital admission. Such patients generally have one or more of the following risk factors.
| Age >60 | Cardiac disease | Diabetes (types 1,2) | Smoker |
| Obesity | Chronic lung disease | Pregnant | Chronic kidney disease |
| Hypertension | Cerebrovascular disease | HIV | Dementia or other mental disorder |
Patients with severe and critical disease should be managed in a hospital. Claims assessors need to be familiar with current practices in their geographies to ensure that approved and paid-for claims for hospital admissions are in keeping with stipulated guidelines.
Treatments: Non-Severe Disease
Treatment mainstays for non-severe disease are outpatient-based care, access to telehealth, and therapies that can provide symptomatic relief. This approach can protect patients, their families, and healthcare workers from COVID-19 infection. Treatment recommendations include:
- Isolation for five to ten days after a positive RT-PCR or antigen test, depending on the country.
- Basic care, e.g., good nutrition, hydration, rest.
- Symptomatic relief using preparations such as antipyretics, antitussives, or expectorants. Oral corticosteroids may also be prescribed for those at higher risks of developing severe disease.
- Oxygenation saturation monitoring, using a pulse oximeter, at least twice a day. If saturation is between 90% and 94%, patients should call a designated hotline, and if less than 90%, should arrange to come to a hospital or other healthcare facility for treatment.
- Supplemental oxygen, either via portable oxygen concentrators or oxygen canisters.
Specific outpatient treatment can also include antiviral agents and monoclonal antibodies. These should be prescribed mostly for patients at risk of progressing to severe disease.
Available pharmaceutical treatments (either FDA-approved or via emergency use authorization [EUA]) include:
- Antiviral agents: These inhibit SARS-COV-2 protein(s) and stop the COVID-19 virus from replicating. They work best for patients with a confirmed diagnosis but who are still early in the disease. Antivirals can usually be identified by the -vir suffix in the drug name. Current options are:
- Nirmatrelvir-ritonavir (Paxlovid) (EUA): Intended specifically for mild to moderate disease, this is to be administered within five days of symptom onset and is to be taken for no more than five days. It is not authorized for pre-exposure or post-exposure prevention, nor for treatment of severe or critical disease.
- Molnupiravir (FDA): Still under consideration by the WHO but is recommended as an alternative by other expert bodies such as NICE UK. It is contraindicated for those under 18 years of age as well as for pregnant patients.
- Remdesivir (FDA): WHO conditionally recommends against using remdesivir for hospitalized patients due to lack of certainty of its benefits. One randomized controlled trial, however, has suggested it be used in outpatient settings for symptomatic patients with risk factors for severe disease. Still, as remdesivir can only be administered intravenously and requires three days to complete the full course, it may not be the most feasible outpatient option.
- Monoclonal antibodies (mAb): These laboratory-produced antibodies target virus proteins and are identified by the -mab suffix in their names. The four mAb preparations developed specifically to target COVID-19 are: bamlanivimab plus etesevimab, which are administered as an infusion; casirivimab plus imdevimab (REGEN-COV), which can be administered as an infusion or a subcutaneous injection, sotrovimab, which is administered intravenously, and the recently approved bebtelovimab, which is administered via infusion. Only sotrovimab and bebtelovimab are found to be effective against the Omicron variant. (The U.S. FDA has withdrawn authorization for REGEN-COV and for bamlanivimab plus etesevimab due to their ineffectiveness against Omicron.)
Treatments: Severe and Critical Disease
The list of mainstay treatments for severe COVID-19 includes: hospitalization; respiratory support; prevention of complications such as anticoagulation prophylaxis; risk factor control; cautious hydration management; and continuation of treatment for existing comorbidities.
- Hospitalization: Given the aggressiveness with which severe COVID-19 needs to be treated, hospitalization is a must, especially for patients with comorbidities that could exacerbate symptoms and predispose them to complications.
- Respiratory support: The majority of hospitalized COVID-19 patients require some form of respiratory support, such as:
- Prone positioning: Lying in a prone position (on one’s abdomen) appears to improve oxygenation and reduces rate of intubation. Patients who meet indications for prone positioning are usually placed on their abdomens for 6 to 12 hours a day.
- Non-invasive ventilation: low- to high-flow oxygen delivered via nasal cannula or a non-invasive ventilation device such as a CPAP machine.
- Invasive ventilation: If noninvasive ventilation does not improve respiration and oxygen saturation, intubation and mechanical ventilation are required. Once intubated, one or more of the several accepted ventilation strategies would be instituted to optimize blood oxygenation.
- ECMO: In extremely severe cases, the patient’s cardiopulmonary system is not able to oxygenate sufficiently to sustain life. Extracorporeal membrane oxygenation (ECMO), which consists of pumping a patient’s blood through a heart-lung machine for oxygenation, and then returning it to the body, is a last resort treatment for these patients. ECMO requires highly skilled experts to administer, and is usually only available in large, specialized healthcare centers.
- Specific inpatient treatments: Anti-inflammatories, antiviral agents, and systemic corticosteroids are recommended in all severe cases.
Anti-inflammatories:
- Dexamethasone: This corticosteroid is used as a systemic anti-inflammatory to counter the high inflammation seen in severe cases. If dexamethasone is unavailable, alternatives such as hydrocortisone, prednisolone, or methylprednisolone can be used.
- Tocilizumab: This monoclonal antibody was originally developed to treat rheumatoid arthritis. It works by blocking the interleukin-6 (IL-6) pathways responsible for inflammation and research has shown it to be effective in controlling inflammation in COVID-19 patients.
- Baricitinib: This kinase (Janus kinase, or JAK) inhibitor was also first developed to treat rheumatoid arthritis. It is thought that baricitinib may also interfere with SARS COV-2 entry into cells.
- Antiviral agents
- Remdesivir: there is still some controversy surrounding the use of remdesivir for hospitalized and severly ill COVID-19 patients. It is recommended by several U.S.-based bodies, such as the Infectious Disease Society of America (IDSA) and the National Institutes of Health (NIH) for individuals on non-invasive ventilation. WHO, however, has recommended against using it because its definitive mortality benefit has not yet been demonstrated. Currently, remdesivir is not recommended for patients requiring invasive ventilation. When faced with differing recommendations by major expert organization such as in this case, claims personnel should review the clinical details of the related claims with their company’s medical team to reach a decision.
Treatments Still Under Investigation
Certain treatment options are still being researched and investigated. Benefits of their use are still unknown or are not supported by conclusive evidence, so they are not recommended, but that could change if evidence of efficacy emerges.
For now, these treatments should be considered investigational or experimental. As such, they are usually excluded by health insurance policies:
- Fluvoxamine: An antidepressant with potential anti-inflammatory effects.
- Convalescent plasma: Plasma derived from recovered patients containing antibodies against SARS-COV-2.
- Inhaled steroids: Corticosteroids delivered via inhalation to reduce inflammatory responses in the lungs and airways. Two – ciclesonide and budesonide – are currently being investigated.
- Systemic corticosteroids: Intravenously delivered corticosteroids for outpatients.
As we enter the third year of the pandemic, the question of whether to cover novel treatments should no longer be an issue. Claims assessors should refer to established treatment guidelines, such as the WHO Living Guidelines, and decline treatments not recommended by these guidelines.
Treatments Not Recommended
These treatments have been studied (some extensively) and have been deemed ineffective or even harmful to patients. Claims assessors should deny payment for the following if prescribed for COVID-19:
- Hydroxychloroquine
- Azithromycin
- Ivermectin
- Colchicine
- Lopinavir-ritonavir
- Sulodexide
- Favipiravir
- Interferons
- IL-1 inhibitors
- Vitamin C, D, and zinc (in excess of
recommended daily nutritional requirements)
Final Thoughts
As COVID-19 continues to evolve and new variants and new evidence emerge, guidelines will continue to update. Claims assessors must be vigilant about keeping up with the latest developments. With the current glut of information now publicly available on COVID-19 treatments, claims assessors must have the tools to appraise all new information carefully.
We suggest using guidelines from expert organizations such as the Centers for Disease Control and Prevention (CDC), the NIH, and the IDSA in the U.S., The National Health Service (NHS) in the U.K., WHO globally, or other established local or regional institutions. When in doubt, do not hesitate to reach out to your company’s medical team for a thorough review.







